HEALTH NEWS - A modified bone marrow transplant procedure for sickle cell disease (SCD) co-developed at Johns Hopkins University School of Medicine, the University of California, San Francisco, and Vanderbilt University Medical Center can cure the disease, a new study has found.
Of 38 adults with severe SCD who participated in the study, more than 97% no longer required immunosuppressive therapy one year after the transplant, the researchers reported Feb. 25 in the journal NEJM Evidence. The two-year survival rate was 95%.
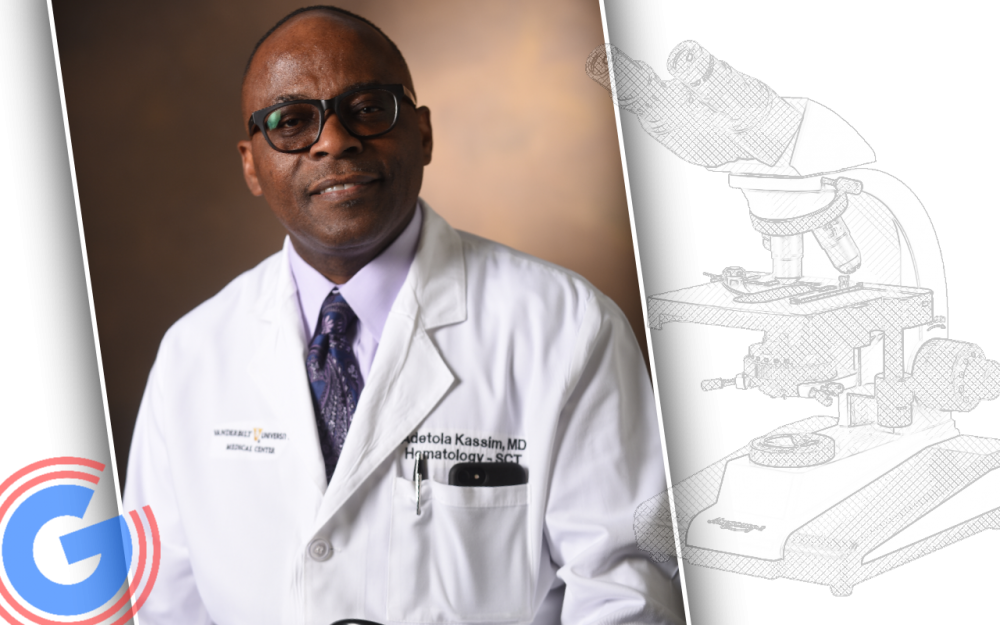
“The findings are significant and have implications for enhancing the care of patients with sickle cell disease,” said Adetola Kassim, MD, MS, professor of Medicine, clinical director of VUMC’s Adult Stem Cell Transplant Program, and co-first author of the paper with Mark Walters, MD, of UCSF.
Results of the phase 2 study, conducted at VUMC and 18 other U.S. centers between 2017 and 2021, echoed the findings of the previously published study — the Vanderbilt-led Global Haploidentical BMT Learning Collaborative — which highlighted a novel conditioning regimen for related HLA-haploidentical bone marrow transplantation (BMT).
“Our earlier clinical trial, published in the journal Blood last year, showed that we can cure SCD in children and adults,” said Michael DeBaun, MD, MPH, the J.C. Peterson, MD, Chair and professor of Pediatrics at VUMC, and director of the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease.
The new trial, conducted through the National Institutes of Health (NIH)-supported Blood and Marrow Transplant Clinical Trials Network, “is a direct extension of our previous study,” DeBaun said. “The results are just as good, if not better, than gene therapy or gene editing therapy.”
More SCD patients with complicating medical conditions should be able to qualify for curative therapy because the new regimen has fewer toxic side effects and is about one-fifth the cost of myeloablative gene therapy, said DeBaun, senior co-author of the paper with Johns Hopkins’ Robert Brodsky, MD, who trained at VUMC.
The study received support from grants U10HL069294 and U24HL138660 provided by the National Heart, Lung, and Blood Institute and the National Cancer Institute of the NIH to the Blood and Marrow Transplant Clinical Trials Network (BMT CTN).